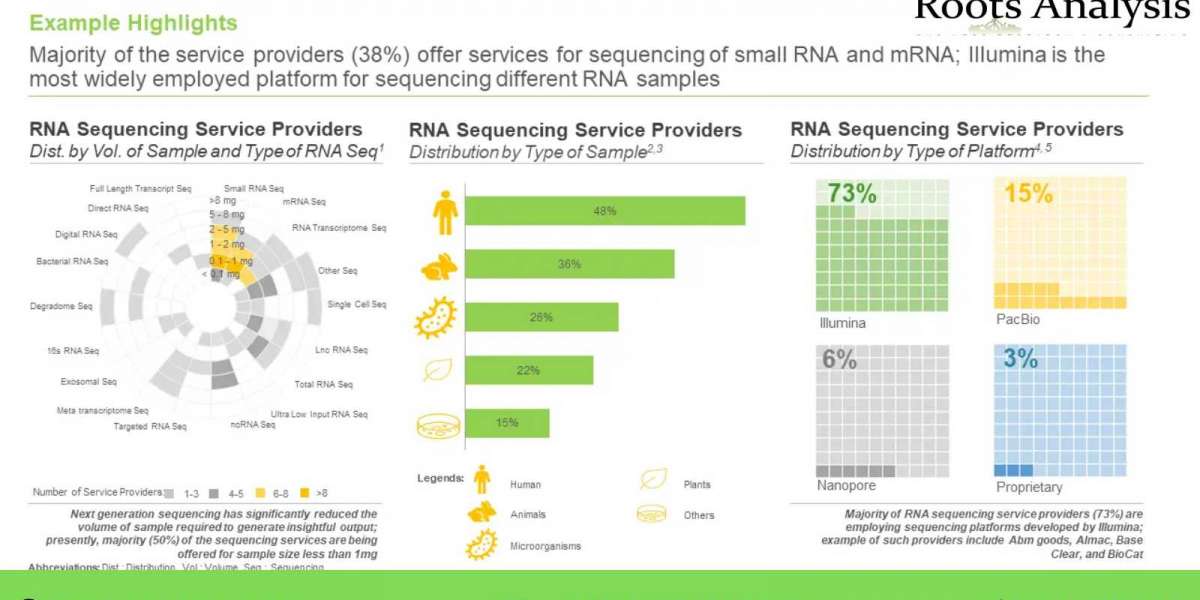
The absence of a crystalline SiO phase under ordinary conditions is an anomaly for the group 14 monoxide sequence. We explore the theoretically ordered ground state and amorphous structure of SiO at P = 1 atm, as well as its crystalline phase at pressures up to 200 GPa. sio compound name Several competing ground-state P = 1 atm structures with Si-Si bonds and Si-O-Si bridges similar to silicon dioxide (SiO2) polymorphs were found. The most stable of these static structures is slightly more stable than the calculated enthalpy of the amorphous SiO random bond model. In that model, we found no segregation into amorphous Si and amorphous SiO2 regions. P = 1 atm The structures are all semiconducting. As the pressure increases, interesting new crystal structures evolve, including Si triangular networks or strips and quartz-like domains. The heat of formation of crystalline SiO was calculated; it was found to be the most negative of all group 14 monoxides. However, given the stability of SiO2, the disproportionation reaction 2SiO(s) → Si(s)+SiO2(s) is exothermic and belongs exactly to the family of group 14 monoxides, ranging from a highly negative disproportionation ΔH towards CO to Highly PbO positive. The heat of disproportionation does not change significantly with pressure, i.e. there is no stable range for SiO relative to SiO2. The high-pressure SiO phase is metallic.
As shown in Figure S4, the 3D structure V contains non-planar 8-
A membered ring consisting of two oxygen atoms and six silicon atoms. These 8-membered rings are
Connections in all three dimensions are shared by Si-O bonds along the a-axis, Si-Si bonds along the c-axis, and Si-Si edge sharing along the b-axis. All silicon atoms in this structure have
Same bonding environment, (Si2O2). The Si-O-Si bonds in this structure are close to
Linear, 173°. As mentioned above, the equistoichiometry of Si and O leads to the formation of
Si-Si bond; this bond is not seen in the SiO2 phase. Si-O and Si-Si bond lengths are 1.65
and 2.38 Å, respectively
Search
Popular Posts
-
 !LINK! Se7en Activa Windows Nulled Activator Exe Ultimate 64bit Full
By alictendi
!LINK! Se7en Activa Windows Nulled Activator Exe Ultimate 64bit Full
By alictendi -
download buku filsafat gratis pdf
By brempecrine -
 Dhoom 2 2006 Utorrent Watch Online Dubbed Subtitles Free Torrent Avi |WORK|
By pornajadeans
Dhoom 2 2006 Utorrent Watch Online Dubbed Subtitles Free Torrent Avi |WORK|
By pornajadeans -
 LAZIO: LE CONDIZIONI DI STEFAN RADU | News – Footb Latest Pc Free Activation Download 32
By bulgambhoca
LAZIO: LE CONDIZIONI DI STEFAN RADU | News – Footb Latest Pc Free Activation Download 32
By bulgambhoca -
 ARTE L’INTEGRITY Torrent Registration Rar Free
By famupartgeb
ARTE L’INTEGRITY Torrent Registration Rar Free
By famupartgeb