If the container of triphenylmethanol in the fire site has changed color or made sound from the safety pressure relief device, it must be evacuated immediately, isolate the accident site, and prohibit irrelevant personnel from entering. Contain and treat fire water to prevent environmental pollution.
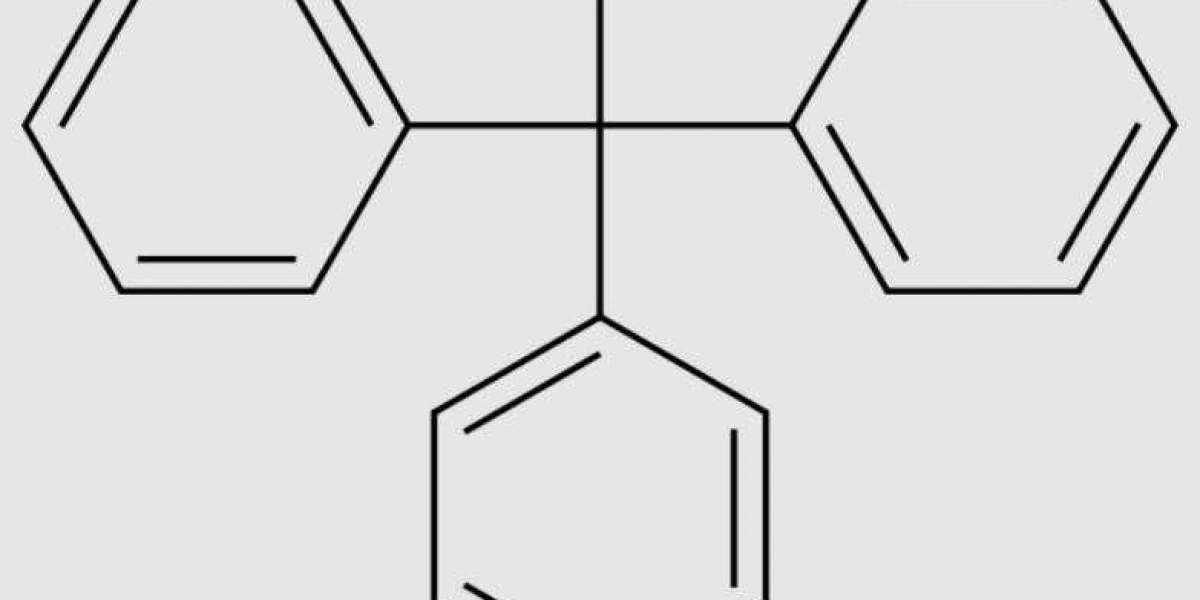
Preparation of triphenylmethanol
Because triphenylmethanol is mainly used in laboratory synthesis of organic reactions, its preparation is also mostly small-scale laboratory preparation. The target product can be obtained by a variety of methods [2-5], the most common of which is the preparation of phenylmagnesium bromide (Grillard's reagent) with benzophenone (or ethyl benzoate), which can also be used in place of benzoate. The detailed steps are as follows: Put phenylmagnesium bromide in a cold water bath, stir the drop funnel and add 1.9ml of ethyl benzoate and 7ml of anhydrous ether mixture, control the drop acceleration to keep the reaction smooth until the addition is complete. Reflux in the water bath (30-35℃) 1h full reaction, there are obvious stratification. After cooling and stirring in ice water bath, saturated ammonium chloride is added to the dripping funnel to dissolve the addition product. The solution is filtered, the filtered solution is taken, and the ether is distilled, cooled and crystallized, and pumped and filtered. triphenylmethanol was obtained by recrystallization with ethanol, extraction and filtration, and drying.
Application of triphenylmethanol
triphenylmethanol itself has no industrial application and is only used in laboratory research, and can be combined with dry hydrogen chloride using ether as a catalyst to produce triphenylchloromethane. Many derivatives of triphenylmethanol are important organic dyes, for example, some of them can be used as intermediates in the synthesis of triphenylmethane dyes.